51. The Daltons law of partial pressure can be written as
52. When two phases α and β in an alloy are in thermodynamic equilibrium, then
53. Consider an ideal solution of component A and B. The entropy of mixing per mole of an alloy containing 50 wt% B, is
54. The hydrogen content of steel in equilibrium with hydrogen gas at 1 bar pressure is 28 ppm at some temperature. Hydrogen content in the metal at the same temperature gets reduced at 1 ppm, when the equilibrium PH2 changes to . . . . . . . . bar.
55. For an ideal gas, Cp - Cv is
56. At 1200°C, the standard Gibbs energy of thermal decomposition of one mole of wustite into Fe and O2 is 168 J.
The corresponding dissociation pressure (in atm.) is
The corresponding dissociation pressure (in atm.) is
57. Uphill diffusion means diffusion from
58. Select the correct plot of Gibbs free energy (G) Vs. Temperature (T) for a single component system from the following:
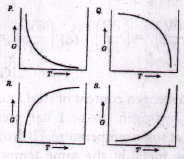

59. The part of the universe excluding the system is known as
60. Reaction between A and B results in an intermediate complex AB* which leads to the final product AB as, A + B ⟶ AB* ⟶ AB. Collision rate theory views the rate as dependent on
Read More Section(Metallurgical Thermodynamics and Kinetics)
Each Section contains maximum 100 MCQs question on Metallurgical Thermodynamics and Kinetics. To get more questions visit other sections.
