1. The decreasing order of nucleophilicity for the following anions is
CH3C$${\text{O}}_2^ - $$, CH3O-, C6H5O-, N$${\text{O}}_3^ - $$
2. The most appropriate sequence of the reactions for carrying out the following conversion

is

is
3. Which of the following compounds is expected to show a sharp singlet for one of its protons at δ ≥ 8 ppm in 1H NMR spectrum, given that this signal remains unaffected on shaking the solution thoroughly with D2O?
4. Conversion of Ph-NH2 into Ph-CN can be accomplished by
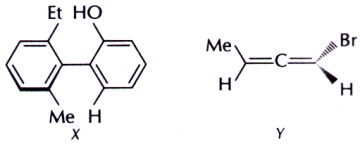
5. The absolute configuration for compounds X and Y respectively are

6. The reactive intermediate involved in the conversion of phenol to salicylaldehyde using chloroform and sodium hydroxide is
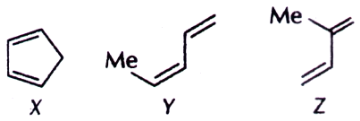
7. Order of reactivity of the following dienes X, Y and Z in Diels-Alder reaction is

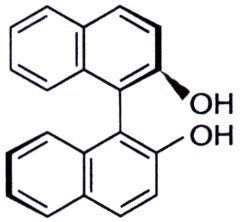
8. The binaphthol (Bnp) is

9. Aniline can be distinguished from methyl amine by its reaction with
10. For the compound,
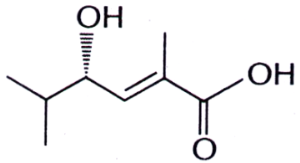
the stereochemical notations are

the stereochemical notations are
Read More Section(Basics of Organic Reaction Mechanism)
Each Section contains maximum 100 MCQs question on Basics of Organic Reaction Mechanism. To get more questions visit other sections.
