If ka ≫ kb, then concentration versus time plot for the reaction is
A. 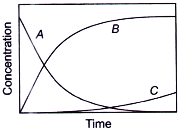
B. 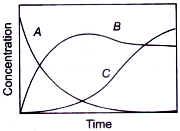
C. 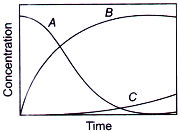
D. 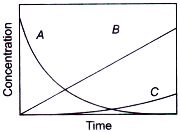
Answer: Option B
Related Questions on Chemical Kinetics
The half-life of a first order reaction varies with temperature according to
A. In t1/2 ∝ 1/T
B. In t1/2 ∝ T
C. t1/2 ∝ 1/T2
D. t1/2 ∝ T2
A. 0.1386 min-1
B. 0.0693 min-1
C. 0.1386 mol L-1min-1
D. 0.0693 mol L-1min-1

Join The Discussion