The configurations at the two asymmetric centres (C-1 and C-6) in the bicyclo [4.4.0] decane, given below are
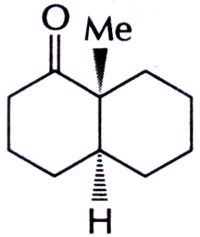
A. 1R, 6R
B. 1R, 6S
C. 1S, 6S
D. None of the above
Answer: Option C
A. CH3C$${\text{O}}_2^ - $$ > CH3O- > C6H5O- > N$${\text{O}}_3^ - $$
B. CH3O- > N$${\text{O}}_3^ - $$ > C6H5O- > CH3C$${\text{O}}_2^ - $$
C. CH3O- > C6H5O- > CH3C$${\text{O}}_2^ - $$ > N$${\text{O}}_3^ - $$
D. C6H5O- > CH3O- > N$${\text{O}}_3^ - $$ > CH3C$${\text{O}}_2^ - $$
The most appropriate sequence of the reactions for carrying out the following conversion

is
A. (i) peracid, (ii) H+, (iii) Zn/dil.HCl
B. (i) Alkaline KMnO4, (ii) NalO4, (iii) N2H4/KOH
C. (i) Alkaline KMnO4, (ii) H+, (iii) Zn/dil.HCl
D. (i) O3/Me2S, (ii) NaOEt, (iii) N2H4/KOH
Conversion of Ph-NH2 into Ph-CN can be accomplished by
A. reaction with sodium cyanide in the presence of nickel catalyst
B. reaction with chloroform and sodium hydroxide
C. diazotisation followed by reaction with CuCN
D. reaction with ethyl formate followed by thermolysis

Join The Discussion