The ionisation energy of hydrogen atom in the ground state is x KJ. The energy required for an electron to jump from 2nd orbit to 3rd orbit is
A. 5x/36
B. 5x
C. 7.2 x
D. x/6
Answer: Option A
Solution(By Examveda Team)
The ionisation energy for the hydrogen atom can be written as:Ionisation energy = energy in first orbit - energy in infinite orbit
Ionisation energy = E = -E0 / n2 , where E0 = 13.6 eV (1 eV = 1.602×10-19 Joules) and n = 1,2,3… and so on.
The difference in energy = Einfinity - E1
so the ionisation energy from the ground state = x KJ = 0-( -E0 /12 ) = E0
The energy required to jump the elctron from second to the third stage is:
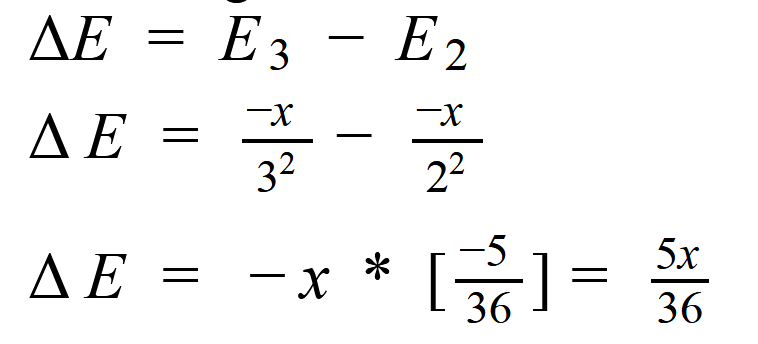
Join The Discussion
Comments ( 2 )
Related Questions on Chemistry
Which of the following phenomenon is considered responsible for Global Warming?
A. Greenhouse effect
B. Fire in coal mines
C. Dry farming
D. Monsoon
E. Trade winds
The nucleus of an atom consists of
A. electrons and neutrons
B. electrons and protons
C. protons and neutrons
D. All of the above
The number of moles of solute present in 1 kg of a solvent is called its
A. molality
B. molarity
C. normality
D. formality

Koi mujhe batayaga iska solution
Solution