Three molecular ionic states, P-R are possible for the amino acid histidine. Identify the correct choice of pH values, respectively for the observation of the ionic states P-R.
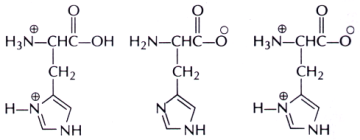
A. P at pH 1; Q at pH 12; R at pH 7
B. P at pH 7; Q at pH 1; R at pH 12
C. P at pH 12; Q at pH 7; R at pH 1
D. P at pH 12; Q at pH 1; R at pH 7
Answer: Option A
A. CH3C$${\text{O}}_2^ - $$ > CH3O- > C6H5O- > N$${\text{O}}_3^ - $$
B. CH3O- > N$${\text{O}}_3^ - $$ > C6H5O- > CH3C$${\text{O}}_2^ - $$
C. CH3O- > C6H5O- > CH3C$${\text{O}}_2^ - $$ > N$${\text{O}}_3^ - $$
D. C6H5O- > CH3O- > N$${\text{O}}_3^ - $$ > CH3C$${\text{O}}_2^ - $$
The most appropriate sequence of the reactions for carrying out the following conversion

is
A. (i) peracid, (ii) H+, (iii) Zn/dil.HCl
B. (i) Alkaline KMnO4, (ii) NalO4, (iii) N2H4/KOH
C. (i) Alkaline KMnO4, (ii) H+, (iii) Zn/dil.HCl
D. (i) O3/Me2S, (ii) NaOEt, (iii) N2H4/KOH
Conversion of Ph-NH2 into Ph-CN can be accomplished by
A. reaction with sodium cyanide in the presence of nickel catalyst
B. reaction with chloroform and sodium hydroxide
C. diazotisation followed by reaction with CuCN
D. reaction with ethyl formate followed by thermolysis

Join The Discussion