Each of the two isolated vessels, A and B of fixed volumes, contains N molecules of a perfect monatomic gas at pressure p. The temperatures of A and B are T1 and T2 respectively. The two vessels are brought into thermal contact. At equilibrium, the change in entropy is
A. $$\frac{3}{2}N{k_B}\ln \left[ {\frac{{T_1^2 + T_2^2}}{{4{T_1}{T_2}}}} \right]$$
B. $$N{k_B}\ln \left( {\frac{{{T_2}}}{{{T_1}}}} \right)$$
C. $$\frac{3}{2}N{k_B}\ln \left[ {\frac{{{{\left( {{T_1} + {T_2}} \right)}^2}}}{{4{T_1}{T_2}}}} \right]$$
D. $$2N{k_B}$$
Answer: Option A
A. $$\frac{1}{{1 + {e^{ - \varepsilon /{k_B}T}}}}$$
B. $$\frac{1}{{1 + 2{e^{\varepsilon /{k_B}T}}}}$$
C. $$\frac{1}{{2{e^{\varepsilon /{k_B}T}} + 4{e^{2\varepsilon /{k_B}T}}}}$$
D. $$\frac{1}{{2{e^{\varepsilon /{k_B}T}} - 4{e^{2\varepsilon /{k_B}T}}}}$$
A. $$\frac{1}{6}{E_F}$$
B. $$\frac{1}{5}{E_F}$$
C. $$\frac{2}{5}{E_F}$$
D. $$\frac{3}{5}{E_F}$$
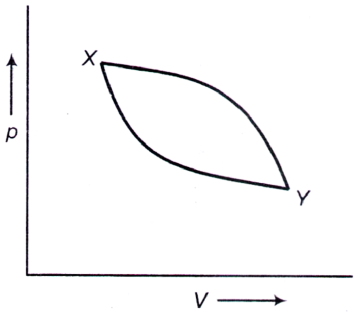
A. clockwise
B. counter-clockwise
C. neither clockwise nor counter-clockwise
D. clockwise from X → Y and counter-clockwise from Y → X
A. $$\exp \left( {\frac{{\hbar \omega }}{{{k_B}T}}} \right) + 1$$
B. $$\exp \left( {\frac{{\hbar \omega }}{{{k_B}T}}} \right) - 1$$
C. $${\left[ {\exp \left( {\frac{{\hbar \omega }}{{{k_B}T}}} \right) + 1} \right]^{ - 1}}$$
D. $${\left[ {\exp \left( {\frac{{\hbar \omega }}{{{k_B}T}}} \right) - 1} \right]^{ - 1}}$$

Join The Discussion