The partition function of two Bose particles, each of which can occupy any of the two energy levels 0 and $$\varepsilon $$ is
A. $$1 + {e^{\frac{{ - 2\varepsilon }}{{kT}}}} + 2{e^{\frac{{ - \varepsilon }}{{kT}}}}$$
B. $$1 + {e^{\frac{{ - 2\varepsilon }}{{kT}}}} + {e^{\frac{{ - \varepsilon }}{{kT}}}}$$
C. $$2 + {e^{\frac{{ - 2\varepsilon }}{{kT}}}} + {e^{\frac{{ - \varepsilon }}{{kT}}}}$$
D. $${e^{\frac{{ - 2\varepsilon }}{{kT}}}} + 2{e^{\frac{{ - \varepsilon }}{{kT}}}}$$
Answer: Option C
A. $$\frac{1}{{1 + {e^{ - \varepsilon /{k_B}T}}}}$$
B. $$\frac{1}{{1 + 2{e^{\varepsilon /{k_B}T}}}}$$
C. $$\frac{1}{{2{e^{\varepsilon /{k_B}T}} + 4{e^{2\varepsilon /{k_B}T}}}}$$
D. $$\frac{1}{{2{e^{\varepsilon /{k_B}T}} - 4{e^{2\varepsilon /{k_B}T}}}}$$
A. $$\frac{1}{6}{E_F}$$
B. $$\frac{1}{5}{E_F}$$
C. $$\frac{2}{5}{E_F}$$
D. $$\frac{3}{5}{E_F}$$
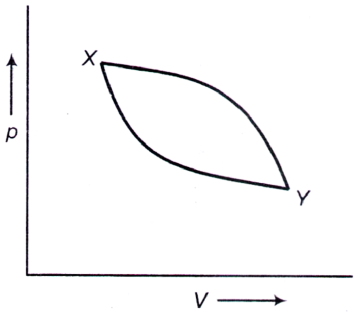
A. clockwise
B. counter-clockwise
C. neither clockwise nor counter-clockwise
D. clockwise from X → Y and counter-clockwise from Y → X
A. $$\exp \left( {\frac{{\hbar \omega }}{{{k_B}T}}} \right) + 1$$
B. $$\exp \left( {\frac{{\hbar \omega }}{{{k_B}T}}} \right) - 1$$
C. $${\left[ {\exp \left( {\frac{{\hbar \omega }}{{{k_B}T}}} \right) + 1} \right]^{ - 1}}$$
D. $${\left[ {\exp \left( {\frac{{\hbar \omega }}{{{k_B}T}}} \right) - 1} \right]^{ - 1}}$$

Join The Discussion