Which among the following sets for Maxwell relation is correct? (U-internal energy, H-enthalpy, A-Helmholtz free energy and G-Gibbs free energy)
A. $$T = {\left( {\frac{{\partial U}}{{\partial V}}} \right)_S}{\text{ and }}p = {\left( {\frac{{\partial U}}{{\partial S}}} \right)_V}$$
B. $$V = {\left( {\frac{{\partial H}}{{\partial p}}} \right)_S}{\text{ and }}T = {\left( {\frac{{\partial H}}{{\partial S}}} \right)_p}$$
C. $$p = - {\left( {\frac{{\partial G}}{{\partial V}}} \right)_T}{\text{ and }}V = {\left( {\frac{{\partial G}}{{\partial p}}} \right)_S}$$
D. $$p = - {\left( {\frac{{\partial A}}{{\partial S}}} \right)_T}{\text{ and }}S = - {\left( {\frac{{\partial A}}{{\partial p}}} \right)_V}$$
Answer: Option B
A. $$\frac{1}{{1 + {e^{ - \varepsilon /{k_B}T}}}}$$
B. $$\frac{1}{{1 + 2{e^{\varepsilon /{k_B}T}}}}$$
C. $$\frac{1}{{2{e^{\varepsilon /{k_B}T}} + 4{e^{2\varepsilon /{k_B}T}}}}$$
D. $$\frac{1}{{2{e^{\varepsilon /{k_B}T}} - 4{e^{2\varepsilon /{k_B}T}}}}$$
A. $$\frac{1}{6}{E_F}$$
B. $$\frac{1}{5}{E_F}$$
C. $$\frac{2}{5}{E_F}$$
D. $$\frac{3}{5}{E_F}$$
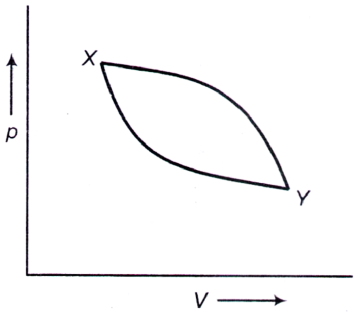
A. clockwise
B. counter-clockwise
C. neither clockwise nor counter-clockwise
D. clockwise from X → Y and counter-clockwise from Y → X
A. $$\exp \left( {\frac{{\hbar \omega }}{{{k_B}T}}} \right) + 1$$
B. $$\exp \left( {\frac{{\hbar \omega }}{{{k_B}T}}} \right) - 1$$
C. $${\left[ {\exp \left( {\frac{{\hbar \omega }}{{{k_B}T}}} \right) + 1} \right]^{ - 1}}$$
D. $${\left[ {\exp \left( {\frac{{\hbar \omega }}{{{k_B}T}}} \right) - 1} \right]^{ - 1}}$$

Join The Discussion